HIGHLIGHTED NEWS
'Bubble Boys' Cured in Medical Breakthrough Using Gene Therapy
April 17 - In a major scientific breakthrough, researchers at St. Jude Children's Research Hospital in Memphis have developed a one-time, personalized treatment used to correct the genetic defect and build fully functioning immune systems in infants with the condition, formally known as X-linked severe combined immunodeficiency, or SCID.
IPTA 2019 - STARTS NEXT WEEK
Onsite registration is still possible directly at the venue as of May 4, 2019.
TTS NEW WEBINAR SERIES - IN CASE YOU MISSED IT
... THE RECORDING IS NOW POSTED

TOPIC: Acute Liver Failure
TITLE: Medical and Surgical Aspects of Acute Liver Failure
TRANSPLANTATION - HIGHLIGHTED ARTICLE
Dr. Joel Thomas Adler, Editorial Fellow, Transplantation
RISK FACTORS AND CLINICAL COURSE FOR LIVER STEATOSIS OR NONALCOHOLIC STEATOHEPATITIS AFTER LIVING DONOR LIVER TRANSPLANTATION
Miyaaki H, Miuma S, Taura N, et al.
Transplantation. 2019 Jan;103(1):109-112
Steatosis occurs frequently in transplanted livers. Miyaaki et al. examined 100 living donor liver transplant recipients and their donors, all of whom had at least one liver biopsy within the first year of transplant. Steatosis in the transplanted liver was identified in 33 cases, and the prevalence was significantly higher (60 vs 23%) among those who received a graft from a donor with steatosis. However, the authors found overall that the clinical course is relatively benign, with only 7.6% achieving any significant fibrosis.
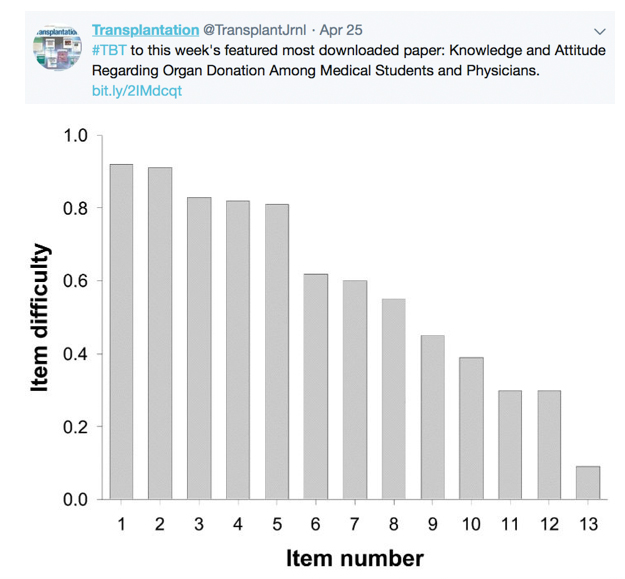
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
Legend: Knowledge items according to item difficulty. Item difficulty (0–1), with lowest values indicating highest difficulty and highest values indicating lowest difficulty. (1) Median transplant-survival in Germany; (2) the possibility of a second transplant after a first has failed; (3) kidney donation in a nonblood relative (e.g., spouse); (4) cadaveric donors as a bigger source for transplant organs in Germany than living donors; (5) percentage of listed patients undergoing transplantation by the end of the year; (6) the median waiting time for transplantation; (7) lifelong immunosuppression to avoid transplant rejection; (8) immunosuppressive therapy and pregnancy; (9) combined kidney and pancreas transplantation in diabetics; (10) influence on quality of life by immunosuppression vs. dialysis; (11) legal aspects of organ donation by living donors in a married couple vs. a nonmarried couple; (12) legal aspects of anonymous living kidney donation; (13) organ explantation in Germany and presumed consent.
Survey on Treatment of Chronic Active Cell-Mediated Rejection for Tx Clinicians and Surgeons - Please Complete!
Please participate in a survey regarding the Treatment of Chronic Activity Cell-Mediated Rejection for transplant clinicians and surgeons. Please click on the link below to complete the survey.
ABSTRACT SUBMISSION NOW OPEN!
The 3rd Joint Meeting of the Turkish Transplantation Society and the Turkic World Transplantation Society will be held in Tashkent, Uzbekistan on October 10-11, 2019. The Scientific and Local Organizing Committees, comprised of international transplantation leaders, have developed a program that reflects current problems and represents a collection of scientific, educational, and practical information. The meeting will be an exciting opportunity for transplant professionals to share their expertise as well as their concerns regarding the development of the field in their own countries. Please visit the website http://tond-tdtd2019.com/en/ for full details.
IXA 2019 - ABSTRACT SUBMISSION DEADLINE EXTENDED TO MAY 12, 2019
ISODP 2019 - DUBAI, UAE - ABSTRACT SUBMISSION DEADLINE EXTENDED TO MAY 19, 2019
IN THE NEWS
Featured Research Liver Frailty Index could identify cirrhosis patients at highest risk of death while waiting for transplants
April 18 - Cirrhosis is a common liver disease, affecting about 1 in 400 adults in the United States annually. Cirrhosis can lead to liver failure or end-stage liver disease, which requires a liver transplant for survival. But up to 25 percent of people who are on waitlists for liver transplants die before receiving one. A new way to measure physical frailty in people with cirrhosis of the liver may help to better identify patients who are most at risk of dying while waiting for a transplant, according to a new study. The research by an NIA- and NIDDK-supported team of investigators was published in the Jan. 17 issue of Gastroenterology.
World’s first lung, liver transplantation in child performed in Russia
April 22 - The world’s first successful combined transplantation of the lungs and liver in a child with cystic fibrosis has been performed at the V.I. Shumakov Medical Research Center in Moscow.
A word of caution on stem cell therapies for pulmonary fibrosis
April 22 - It is widely recognized that the field of regenerative medicine, which includes cell-based therapies such as stem cell treatments, holds a lot of potential for the treatment of pulmonary fibrosis. There continues to be extensive research into stem cell therapies to treat this patient population. However, these new treatments require evaluation and approval of efficacy and safety through rigorous clinical trials and regulatory review. Unfortunately, there are for-profit stem cell centres where practitioners offer unapproved stem cell therapies. These unproven treatments are being given at unregulated centres and may be putting pulmonary fibrosis patients at risk.
U.S. hospitals sue over new national liver transplant policy
April 23 - Hospitals and patients in the United States have sued to block a new nationwide liver transplant policy that they say will waste viable livers, lead to fewer transplants and likely cause deaths.
UPCOMING MEETINGS AND ANNOUNCEMENTS
IPITA 2019 LYON - FRANCE
Registration is open and preliminary program are both available.
2019 TTS TRANSPLANTATION SCIENCE COMMITTEE NEWS
HOLD THE DATE - NOVEMBER 10-13, 2019
ITS 2019 is set for Nov. 10-13 in Clearwater Beach, Florida. Keynote speakers include Katherine High, President and Head of R&D at Spark Therapeutics, and Ronald Germain, chief of Laboratory of Immune System Biology and Lymphocyte Biology Section at the National Institute of Allergy and Infectious Diseases National Institutes of Health.

TID2019 - SAVE THE DATES!
PRE-MEETING TO ISODP 2019 - SAME VENUE ...ONE DAY PRIOR!
Contact
+1-514-874-1717
This email address is being protected from spambots. You need JavaScript enabled to view it.
Address
The Transplantation Society
International Headquarters
505 Boulevard René-Lévesque Ouest
Suite 1401
Montréal, QC, H2Z 1Y7
Canada